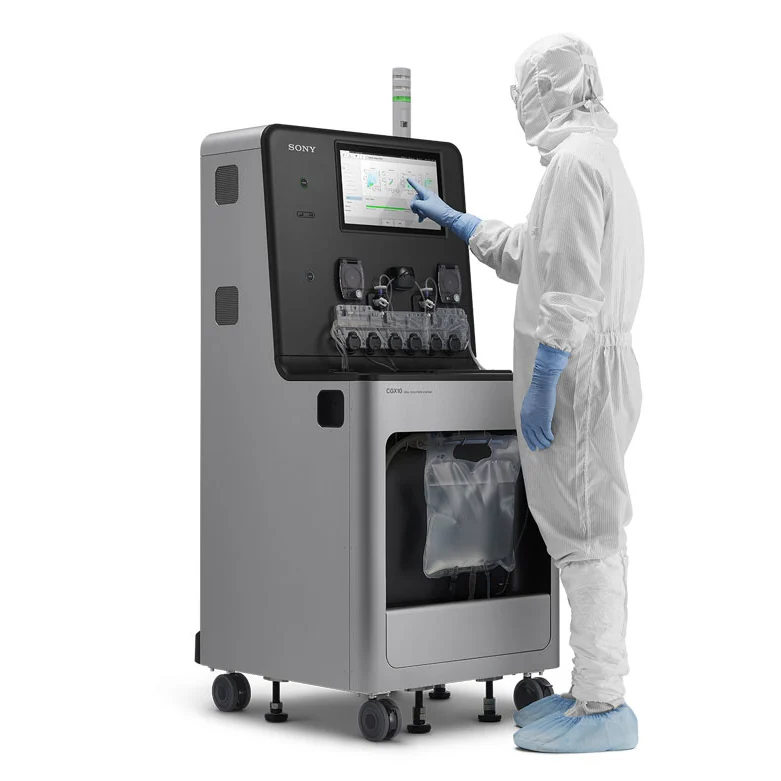
The CGX10 Cell Isolation System represents a breakthrough in the field of cell production and sorting, specifically designed to meet the rigorous demands of GMP-compliant environments. As the only fully closed cell isolation system available, the CGX10 sets a new standard for cell sorting applications, combining superior isolation capabilities with user-friendly operation and unmatched flexibility. This system is particularly valuable in the rapidly advancing fields of cell and gene therapies, where precise cell sorting and isolation are critical for the development of effective treatments.
Innovative Design for GMP Compliance
One of the key advantages of the CGX10 Cell Isolation System is its fully closed design, which is essential for maintaining GMP compliance during cell production processes. Traditional flow cytometers, which have been widely used in research laboratories and early-phase clinical studies, often fall short in GMP environments due to their complex design and the potential for inducing stress or damage to sorted cells. The CGX10 overcomes these limitations by providing a robust, closed system that minimizes the risk of contamination and ensures the highest cell viability during the sorting process. This makes it an ideal choice for environments where strict regulatory standards must be met.
Superior Cell Isolation with Multi-Marker Selection
The ability to isolate cells based on multiple biomarkers is a crucial feature of the CGX10 system. In cell and gene therapies, it is often necessary to identify and isolate specific immune cells that exhibit the desired phenotype, which may be defined by the presence of one or more biomarkers. Multi-marker-based cell isolation not only enhances the purity of the isolated cells but also allows for the selective elimination of non-target cells, such as over-differentiated T cells, which may be therapeutically ineffective. By ensuring that only the most relevant cells are selected, the CGX10 improves the overall efficacy of cell-based therapies.
In contrast to traditional flow cytometers, which are typically limited in their ability to handle complex multi-marker sorting in a GMP setting, the CGX10 is designed to excel in these environments. Its advanced technology allows for precise, high-purity isolation of target cells while maintaining their viability, making it a critical tool for researchers and manufacturers involved in the development of advanced therapy medicinal products (ATMPs), including cell and gene therapies.
Streamlined Operation for Efficient Cell Production
Ease of operation is another hallmark of the CGX10 Cell Isolation System. The system is designed to provide a seamless user experience, making it accessible to trained laboratory technicians who may not have specialized expertise in flow cytometry. This is particularly important in GMP-compliant environments, where the complexity and subjectivity of conventional sorter platforms can be a significant barrier to efficient cell production. The CGX10 simplifies the cell isolation process, reducing the need for extensive operator training and minimizing the potential for user error.
The system’s intuitive interface and automated processes allow for a smooth transition from process development to full-scale cell production. This flexibility is essential for laboratories and manufacturing facilities that are scaling up their operations to meet the growing demand for cell-based therapies. By streamlining the workflow, the CGX10 enables researchers and manufacturers to focus on the critical aspects of their work, such as optimizing cell culture conditions and ensuring the quality of the final product.
Application in Advanced Cell and Gene Therapies
The CGX10 Cell Isolation System is specifically designed for use in research, process development, and manufacturing environments related to advanced therapy medicinal products (ATMPs), including cell and gene therapies. These fields have seen significant progress in recent years, driven by the need to develop more effective treatments for a range of diseases. The ability to isolate specific cell populations with high precision is a key factor in the success of these therapies, and the CGX10 provides the tools needed to achieve this.
It is important to note that the CGX10 and its related products are intended for ex vivo cell separation processing only. They are not designed for therapeutic, diagnostic, or in vivo applications in humans. Any clinical use of the cells processed with the CGX10 must adhere to the relevant regulations, and it is the responsibility of the user to ensure compliance with these standards. The CGX10 system is not sold as a medical device, and its use in clinical applications is subject to the appropriate regulatory approvals.
Ensuring the Highest Standards of Cell Production
In the context of GMP-compliant cell production, the CGX10 Cell Isolation System offers unparalleled reliability and performance. Its closed design, multi-marker selection capabilities, and user-friendly operation make it an ideal choice for laboratories and manufacturing facilities that require precise, high-purity cell sorting. As the demand for cell and gene therapies continues to grow, the CGX10 provides the flexibility and efficiency needed to support the development of these innovative treatments.
Moreover, the system’s ability to maintain high cell viability throughout the sorting process is crucial for the success of cell-based therapies. By minimizing the potential for stress or damage to the sorted cells, the CGX10 ensures that the final product is of the highest quality, which is essential for both research and clinical applications. This makes the CGX10 an invaluable tool for advancing the field of regenerative medicine and for bringing new therapies to market.
Conclusion
The CGX10 Cell Isolation System represents a significant advancement in the field of cell sorting and isolation, particularly in the context of GMP-compliant environments. Its fully closed design, multi-marker selection capabilities, and streamlined operation set it apart from traditional flow cytometers, making it the ideal choice for laboratories and manufacturing facilities involved in the development of advanced cell and gene therapies. As the only fully closed system available for GMP-compliant cell production, the CGX10 offers the reliability, flexibility, and ease of use needed to support the growing demand for high-quality cell-based therapies.
In summary, the CGX10 Cell Isolation System is not just a tool for cell sorting; it is a comprehensive solution for researchers and manufacturers working at the forefront of regenerative medicine. With its ability to deliver high-purity, viable cells in a GMP-compliant environment, the CGX10 is poised to play a crucial role in the advancement of cell and gene therapies, ultimately improving patient outcomes and driving innovation in the field of medicine.